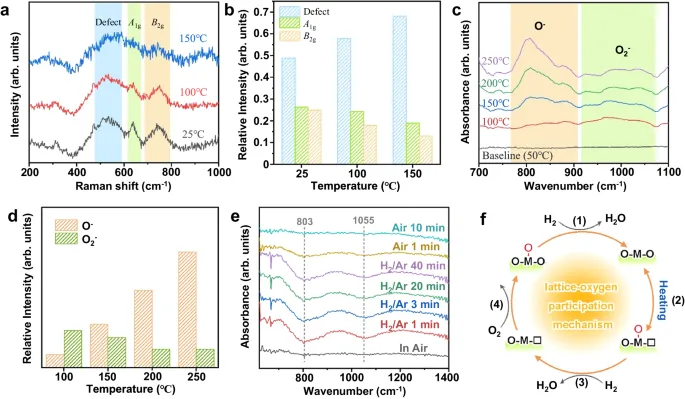
Understanding the sensing mechanism of metal oxide semiconductors is imperative to the development of high-performance sensors. The traditional sensing mechanism only recognizes the effect of surface chemisorbed oxygen from the air but ignores surface lattice oxygen. Herein, using in-situ characterizations, we provide direct experimental evidence that the surface chemisorbed oxygen participated in the sensing process can come from lattice oxygen of the oxides. Further density functional theory (DFT) calculations prove that the p-band center of O serves as a state of art for regulating the participation of lattice oxygen in gas-sensing reactions. Based on our experimental data and theoretical calculations, we discuss mechanisms that are fundamentally different from the conventional mechanism and show that the easily participation of lattice oxygen is helpful for the high response value of the materials.