Rong-Lin Zhong* and Shigeyoshi Sakaki* J. Am. Chem. Soc. 2020, 142, 39, 16732–16747 https://doi.org/10.1021/jacs.0c07239
a.Laboratory of Theoretical and Computational Chemistry, Institute of Theoretical Chemistry, College of Chemistry, Jilin University, Changchun 130023, P. R. China;
b.Element Strategy Initiative for Catalysts and Batteries, Kyoto University, Nishikyo-ku, Kyoto 615-8245, Japan.
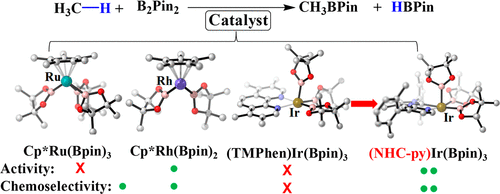
Methane borylation catalyzed by Cp*M(Bpin)n (M = Ru or Rh; HBpin = pinacolborane; n = 2 or 3) and (TMPhen)Ir(Bpin)3 (TMPhen = 3,4,7,8-tetramethyl-1,10-phenanthroline) was investigated by DFT in comparison with cyclohexane borylation. Because Ru-catalyzed borylation has not been theoretically investigated yet, its reaction mechanism was first elucidated; Cp*Ru(Bpin)31-Ru is an active species, and Cp*Ru(Bpin)3(H)(CH3) 4-Ru is a key intermediate. In 4-Ru, the Ru is understood to have an ambiguous oxidation state between +IV and +VI because it has a H··Bpin bonding interaction with a bond order of about 0.5. Methane borylation occurs through oxidative addition of methane C–H bond followed by reductive elimination of borylmethane in all of these catalysts. The catalytic activity for methane borylation increases following the order Cp*Ru(Bpin)3 < (TMPhen)Ir(Bpin)3 < Cp*Rh(Bpin)2. Cyclohexane borylation occurs in the same mechanism except for the presence of isomerization of a key intermediate. Chemoselectivity of methane over cyclohexane increases following the order Ir < Ru < Rh. In all of these catalysts, the rate-determining step (RDS) of cyclohexane borylation needs a larger ΔG°‡ than the RDS of methane borylation because the more bulky cyclohexyl group induces larger steric repulsion with the ligand than methyl. One reason for the worse chemoselectivity of the Ir catalyst is its less congested transition state of the reductive elimination of borylcyclohexane. Herein, use of a strongly electron-donating ligand consisting of pyridine and N-heterocyclic carbene with bulky substituents is computationally proposed as a good ligand for the Ir catalyst; actually, the Ir complex of this ligand is calculated to be more active and more chemoselective than Cp*Rh(Bpin)2 for methane borylation.


